
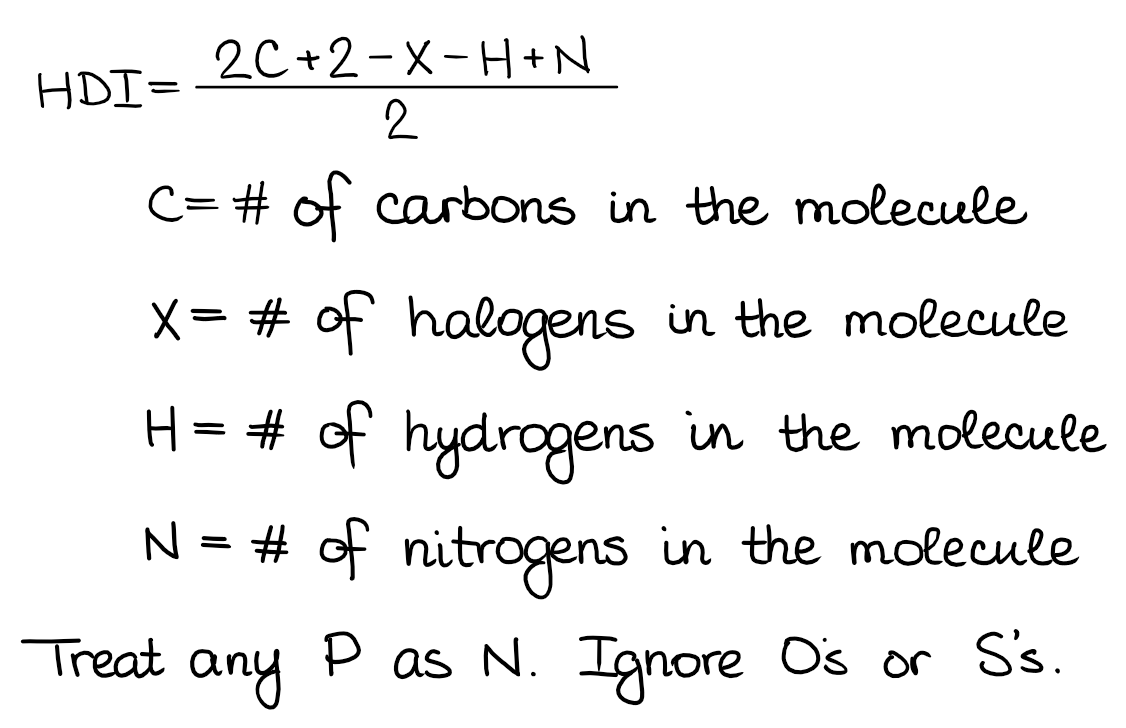
One degree of unsaturation is equivalent to 1 ring or 1 double bond (1 \pi bond).Each row corresponds to a different combination. The following chart illustrates the possible combinations of the number of double bond(s), triple bond(s), and/or ring(s) for a given degree of unsaturation. As seen in alcohols, the same number of hydrogens in ethanol, C 2H 5OH, matches the number of hydrogens in ethane, C 2H 6. Oxygen and sulfur are not included in the formula because saturation is unaffected by these elements. This can be seen with C 3H 9N compared to C 3H 8. Therefore, we add the number of nitrogens (N). For instance, in chloroethane, C 2H 5Cl, there is one less hydrogen compared to ethane, C 2H 6.įor a compound to be saturated, there is one more hydrogen in a molecule when nitrogen is present. The formula subtracts the number of X’s because a halogen (X) replaces a hydrogen in a compound.
Degrees of unsaturation is equal to 2, or half the number of hydrogens the molecule needs to be classified as saturated. The compound needs 4 more hydrogens in order to be fully saturated (expected number of hydrogens-observed number of hydrogens=8-4=4). Thus, the number of hydrogens can be represented by 2C+2, which is the general molecular representation of an alkane. As an example, for the molecular formula C 3H 4 the number of actual hydrogens needed for the compound to be saturated is 8. Another way of interpreting this is that a saturated molecule has the maximum number of hydrogen atoms possible to be an acyclic alkane. X is the number of halogens (F, Cl, Br, I)Īs stated before, a saturated molecule contains only single bonds and no rings.If the molecular formula is given, plug in the numbers into this formula: Calculating The Degree of Unsaturation (DoU)


 0 kommentar(er)
0 kommentar(er)
